Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Initialising ...
Fuji, Hiroyuki*; Aoki, So; Ishii, Tomohiro*; Sakai, Junichi*
Zairyo To Kankyo, 64(5), p.178 - 182, 2015/05
This study focused on a breakdown of passive film which is followed by rust staining, and the objective of this study was to clarify the effect of stability of passive film on the resistance of rust staining of stainless steels. Atomospheric exposure test was carried out for 12 months. In order to compare the stability of passive film, measurements of potential-decay curves, and potentiostatic polarization tests were performed in acidic aqueous chloride solution. As a result, rust area of austenitic stainless steel was higher than that of ferritic stainless steel. This order didn't follow the orders of pitting potentials and densities of inclusions on surface between specimens. On the contrary, the order of the resistance of rust staining of stainless steels followed the order of the stability of passive film. One of the reasons why the resistance of rust staining of austenitic stainless steel was worse than that of ferritic stainless steel was seemed that chloride more easily broke passive film on the surface of austenitic stainless and formed micro pits which become initiations of rust staining and increase density of stains.
Sumiyama, Morio*
JNC TJ8400 2000-009, 138 Pages, 2000/02
To evaluate corrosion behavior of carbon steel, a candidate materials of overpack, buried in soil for a long time, the water pipes buried in freshwater clay for a long time we digged out and the soil environment and the corrosion weight loss of pipes have been researched. From the results, a corrosion model (an empirical equation), an oxygen reduction reaction rate-determing step type, of carbon steel buried in soil was introduced. The corrosion data of under ground pipe collected by the Japan Community Gas Associations was used to increase reliability of the corrosion model equation. These data are one of researches of corrosion behavior of carbon steel buried in soil for a long time studied by at home and abroad. 38 samples buried freshwater clay were selected in 171 samples. With estimating the corrosion velocities and the soil environment factors of the above data, the maximum depth of pit corrosion was calculated by the statistical method of the extreme values using the area of overpack as the recurrent time. The correlation between the soil environment factors and the corrosion weight loss was obtained by the correlation analysis. The corrosion model of the maximum depth of pit corrosion at 0.99 of cumulative probability was compared between the under ground pipe data and the above data. On the reference data and the above data, the corrosion model equation; H = aY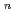 was compared with the maximum depth of pit corrosion at 0.99 cumulative probability. The data of water pipes and community gas pipes at 0.99 cumulative probability showed the reasonable values when these data were compared with the reference data. So that the model was proved as a good corrosion model m the neutral low dissolved oxygen environment.
was compared with the maximum depth of pit corrosion at 0.99 cumulative probability. The data of water pipes and community gas pipes at 0.99 cumulative probability showed the reasonable values when these data were compared with the reference data. So that the model was proved as a good corrosion model m the neutral low dissolved oxygen environment.
; Yoshida, Eiichi; Furukawa, Tomohiro; Aoto, Kazumi
PNC TN9410 97-092, 87 Pages, 1997/07
This experiment is carried out in the series of the investigation on the damage mechanism of carbon steel. In this paper, the damage situation is considered by structure observations. The test were carried out in 600 C-1200
C-1200 C temperature range, in blowing an argon gas. The reagents are Na
C temperature range, in blowing an argon gas. The reagents are Na O, Na
O, Na O
O and NaOH. From structure observations, the holes are observed on the surface of iron-base material in some test conditions. This result is indicated that the selective reaction occurs. The selective reaction is more obvious as the time exposed to the high temperature is longer. It is considered that the selective reaction occurs after the chemical reaction between iron-base material and sodium compound. The areas, in which Mn-concentration is higher, are observed in products on the surface of specimen.
and NaOH. From structure observations, the holes are observed on the surface of iron-base material in some test conditions. This result is indicated that the selective reaction occurs. The selective reaction is more obvious as the time exposed to the high temperature is longer. It is considered that the selective reaction occurs after the chemical reaction between iron-base material and sodium compound. The areas, in which Mn-concentration is higher, are observed in products on the surface of specimen.
; ; Nekoya, Shinichi; ;
JAERI-M 85-070, 34 Pages, 1985/06
no abstracts in English